The Nitrogen Cycle
Overview
When we talk about having a cycled and established tank, we are really referring to having a tank with a developed and functioning nitrogen cycle within our aquarium. Before you cycle an aquarium for the first time, it would benefit you to better understand how the nitrogen cycle works.
You can find examples of the nitrogen cycle everywhere in nature, on land, in our lakes, and in our oceans. Nitrogen is a very important substance which is used by living organisms to sustain life. Essentially, various plants, algaes, and other living organisms consume/use nitrate to grow. Smaller organisms will consume these algaes and other organism as their main food source. These living organisms will be consumed by larger organisms,… and so on. The waste produced by all these organisms will become Ammonia (NH3) and this will broken down back into nitrates by different bacterias so the cycle will continue. The decaying plant and organic matter (decaying organisms that have died) will also produce ammonia required for the nitrogen cycle
Ammonia is toxic to most all living things. The naturally growing bacteria that will consume this ammonia will do so almost instantly removing the ammonia as it is producted before it can harm other organisms. The waste this bacteria produces is nitrites (NO2). Nitrites are also toxic, but not as bad as ammonia. There will be other types of naturally occurring bacteria that will quickly consume the nitrites and the waste produced by this bacteria will be in the form of nitrates (NO3) starting the whole cycle over again. The below diagram will illustrate this for you a little better than I described above.
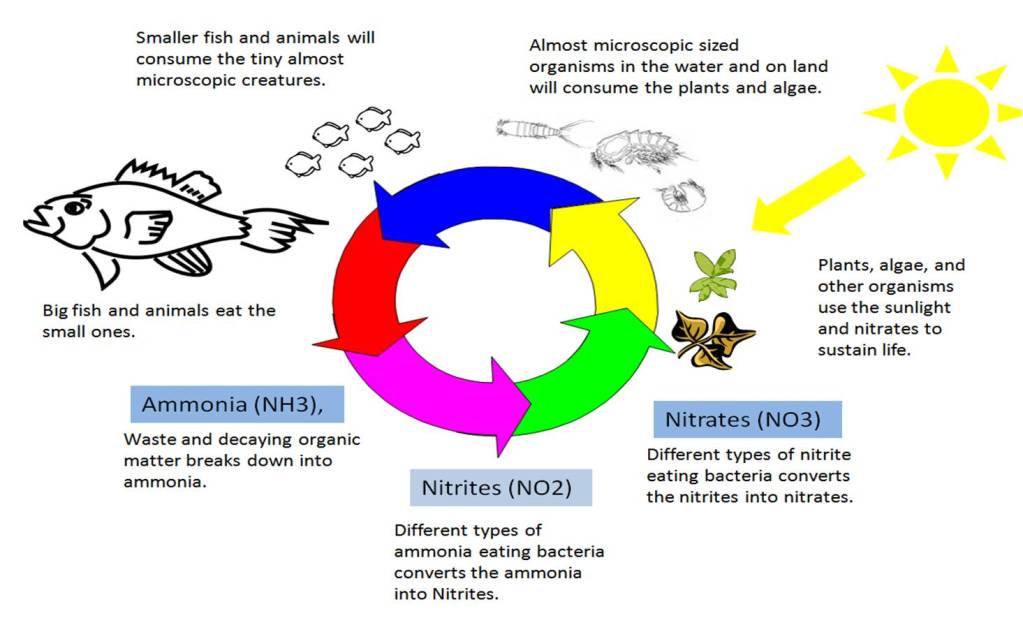
Obviously, this is a very simplified explanation and diagram to give you a good understanding of some of the basic concepts of how the nitrogen cycle works. This can help you to better understand what is going on in your tank.
The nitrogen cycle – Overview of what happens in your marine aquarium
What happens in nature basically happens in you tank. The biggest difference is that since our tanks are a much smaller version of the world around us, so it can be greatly affected by even some of the smallest changes. With such a small ecosystem (our aquariums that is) filled with more fish and critters in comparison to the ecosystem of an ocean, the effects of waste produced can become amplified when the nitrogen cycle is not able to handle the waste. As your tank is a closed system, all the waste (ammonia) stays in the tank unless you adapt the system to handle the amount of waste being produced. This can make achieving a balance ecosystem challenging at times. The below illustration adds a little more detail to the nitrogen system in an aquarium.
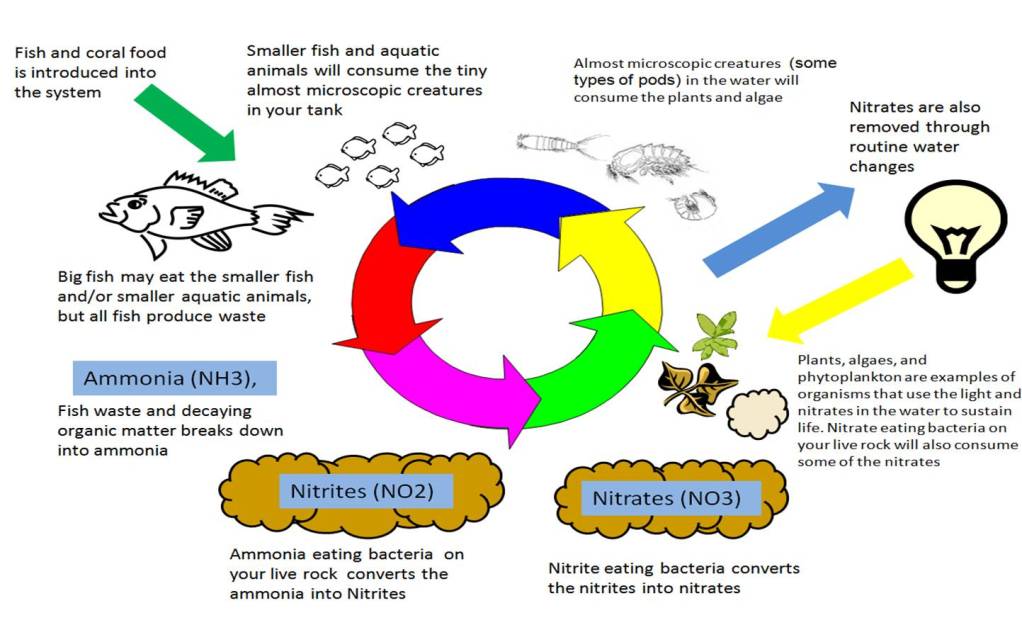
This is where using some rules of thumb can come into play. For example, most people will tell you that you needed a minimum of 1 lb of good quality live rock for each gallon of water. The reason being is that live rock provides a good environment for both nitrifying and denitrifying bacteria to grow. Live rock has many nooks and crannies of porous surfaces that provide a lot of places for these bacteria to grow. The larger the amount of nitrifying and denitrifying bacteria that you can grow, the easier it will be to achieve a balanced ecosystem in your aquarium.
Cycling your tank is just another way of saying you are establishing the nitrogen cycle in your aquarium. We start by providing the right environment and large amounts of surface area (live or dry rock) for the bacteria to grow. We add a source of waste (ammonia) for the ammonia eating bacteria to grow. As they grow they produce nitrite providing a food source for the nitrite eating bacteria grow. As the nitrite eating bacteria grow, they will produce a food source (nitrate) for the nitrate eating bacteria to grow as well as providing algaes and other micro organisms some nitrates to live. Once this has been completed, we have cycled the tank and start to add fish and invertebrates which will provide a source of waste for the cycle to continue. We will then make adjustments along the way increasing and/or decreasing the variables which effect the nitrogen cycle (ie… the amount of fish, the amount of live rock, the amount of routine water changes…. (and so on) to better balance the nitrogen cycle in our tanks
The nitrogen cycle – Overview of what happens in your freshwater aquarium
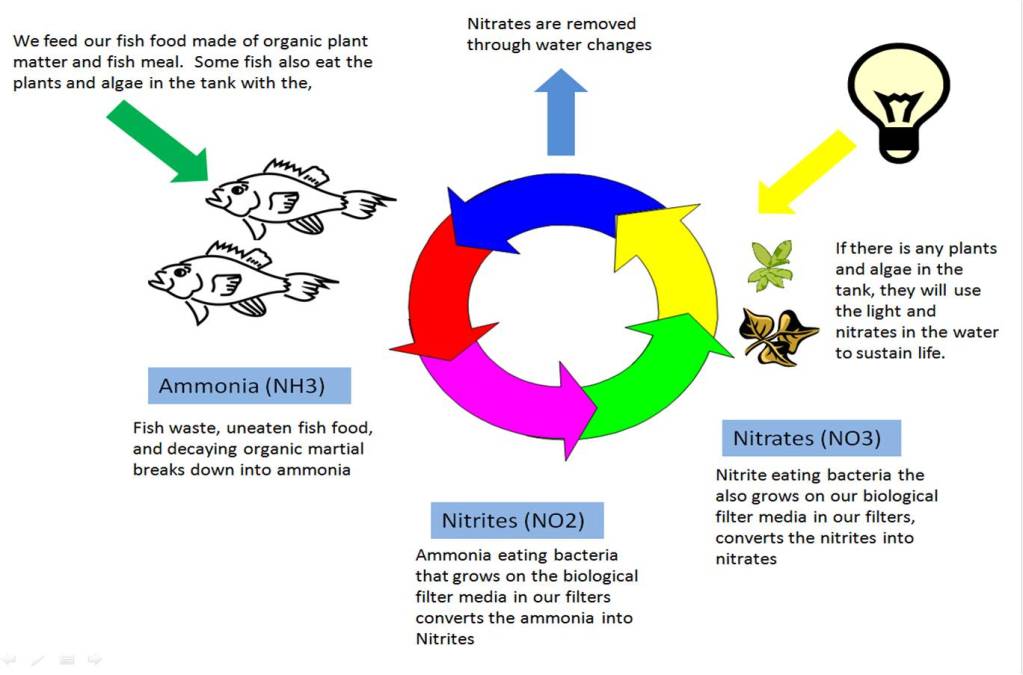
As most people who choose to keep marine aquariums have some experience with fresh water aquariums, I felt it would be useful to explain some of the little differences between the two. As with the marine environment, the same concepts apply to the fresh water environments. That is why there are a lot of similarities between the nitrogen cycle in marine aquariums and fresh water aquariums. Although there are different algaes at work, the algaes in the marine nitrogen cycle and the bacteria in the fresh water nitrogen cycle perform the same functions. This biggest difference is that you will not naturally grow the nitrogen eating bacteria in a fresh water environment.
The below diagram shows the fresh water nitrogen cycle at work. As you can see, the basics of the two different environments are the same.
The below article can provide you with more details on how to cycle a marine aquarium
I really liked your blog post. Really Great.
Get the very biggest setysm you can afford, (not the just biggest tank but parts to match), a larger water volume is more stable. You need to get a tank that is deep than it is tall otherwise your tank will look as if all the liverock is right a the front of the tank.Juwel tanks are excellent quality. Don’t you a secondhand tank unless you are sure of its history, copper based freshwater meds will kill the marine inverts.